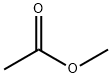
The application of Methyl acetate
General description
Methyl acetate appears as a clear colorless liquid with a fragrant odor. Moderately toxic. Flash point 14°F. Vapors heavier than air. Methyl acetate is an acetate ester resulting from the formal condensation of acetic acid with methanol. A low-boiling (57 ℃) colourless, flammable liquid, it is used as a solvent for many resins and oils. It has a role as a polar aprotic solvent, a fragrance and an EC 3.4.19.3 (pyroglutamyl-peptidase I) inhibitor. It is an acetate ester, a methyl ester and a volatile organic compound. Methyl acetate is a natural product found in Peristeria elata, Coffea arabica, and other organisms with data available[1].
Application and Pharmacology
Methyl acetate (methylacetate), also known as methyl acetate, as an unrestricted organic pollutant discharge, can meet environmental protection standards, and gradually replace solvents such as acetone, butanone, ethyl acetate and cyclopentane. In addition, methyl acetate is also an important basic organic chemical raw material, which can be used as an intermediate for the production of medicines and pesticides, and is also the main raw material for the production of food additives, coatings, inks, resins, adhesives and paints.
1. Methyl acetate arrests Th1 in peripheral immune system and alleviates CNS inflammation in EAE:Multiple sclerosis (MS) is an inflammatory autoimmune disease of the central nervous system (CNS) mediated by immune cells. The pathogenesis of most autoimmune diseases has some degree of similarity to that of MS, and therefore the study of MS has clinical and scientific significance for other autoimmune diseases as well. As a widely used organic solvent, Methyl Acetate (MA) has a similar structure to acetate which has been shown to be therapeutic in the mouse model of multiple sclerosis. Here we found that MA was effective in reducing the disease severity of Experimental Autoimmune Encephalomyelitis (EAE). Pathological sections showed that MA reduced inflammatory cell infiltration in the CNS and attenuated demyelination in the spinal cord. MA increases the proportion of Th1 cells in the periphery of EAE mice. Further mechanistic studies have demonstrated that MA treatment induces Th1 retention in the peripheral immune system by increasing the expression levels of peripheral Th1-related chemokines CXCR3. CXCL9, CXCL10. In addition, we observed that MA alleviated intestinal inflammation in EAE mice. The data showed that this phenomenon is achieved by enhancing IL-10 and inhibiting IL-6 secretion. Our data indicates that MA might have therapeutic implications for autoimmune diseases such as MS[2].
2. A highly volatile floral semiochemical mediating specialized plant‑beetle interactions:Olfactory signaling is key to the reproductive biology of entomophilous palms. Both pollinating and specialized herbivorous insects are attracted to fragrance-emitting palm inflorescences that function as reliable food sources, as well as mating and oviposition sites. In the present study, we characterized the floral scent chemistry of the acuri palm (Attalea phalerata), assessing its role in the attraction of flower-visiting insects associated with this species over its natural distribution range. Sampled insects from staminate inflorescences of A. phalerata (n = 6) at four different sites in the Brazilian Atlantic Forest and Cerrado, and Colombian Amazon basin. Dynamic headspace scent samples of both pistillate and staminate inflorescences of A. phalerata (n = 3♀, 3♂) were collected and analyzed by gas chromatography-mass spectrometry. Methyl acetate, a rare floral scent compound, was identified as the almost exclusive constituent (> 99.8% relative percentage) in all the samples. Flight-interception traps baited with methyl acetate, installed in one of the sites in the Brazilian Cerrado, were attractive to beetles associated with inflorescences of A. phalerata across all four sampling sites (9 spp. in total), including the putative main pollinators (Mystrops spp., Nitidulidae; Andranthobius spp., Curculionidae) and various palm borers (Paratenthras martinsi, Cerambycidae; Parisoschoenus sp.1 and Belopoeus sp.1; Curculionidae). Methyl acetate is highly volatile and we hypothesize its efficacy relies on profuse emission by the inflorescences of A. phalerata, as specialized pollinating insects respond to high concentrations of the attractant, perhaps before odor plumes rapidly disperse. Such a strategy could prove particularly effective in dense populations of A. phalerata[3].
Figure 1 the application of Methyl acetate
Synthesis
At present, acetate esterification is the main method for synthesizing methyl acetate. Acetic acid and methanol are catalyzed by a homogeneous catalyst (sulfuric acid) to obtain methyl acetate through an esterification reaction. The method has disadvantages such as serious corrosion of equipment by sulfuric acid, low conversion rate and long reaction time. In addition, methyl acetate can form an azeotrope with water or methanol, which is difficult to separate, and it is difficult to obtain high-purity methyl acetate. Therefore, in recent years, the sulfuric acid catalyst in the methyl acetate synthesis process has been gradually replaced by catalysts such as solid acid catalysts, ionic liquids and heteropolyacids. In addition, the continuous improvement of the production process has promoted the progress of the technology of synthesizing methyl acetate by the acetic esterification method in my country. Cation exchange resin solid acid was used as catalyst, acetic acid and methanol were used as raw materials, and methyl acetate was synthesized by means of reactive distillation coupled with molecular sieve adsorption and dehydration coupling. The results show that under the condition that the mass fraction of 731 cation exchange resin is 4%, n (acetic acid):n (methanol) = 3:1, reflux ratio (R) is 0.8, and reaction time is 7h, the conversion rate of methanol reaches After the catalyst was reused 6 times, the conversion rate of methanol could still reach 98.9%, and the mass fraction of methyl acetate was still higher than 99.4%.%. Chromatography shows that the product is almost free of by-products and the reaction is highly selective[4].
Safety
1.Health Hazards: Has anesthetic and stimulating effects. Exposure to the vapor of this product causes eye burning, tearing, progressive dyspnea, headache, dizziness, palpitations, depression, and central nervous system depression. The methanol produced by its decomposition can cause vision loss, narrowing of the visual field and optic atrophy.
2. Toxicological data and environmental behavior Acute toxicity: LD505450mg/kg (oral in rats); 3700mg/kg (oral in rabbits) Irritation: Eyes in rabbits: 100mg, moderate irritation. Rabbit percutaneous open stimulation test: 360mg, mild stimulation. Mutagenicity: Deletion and nondisjunction of sex chromosomes: Saccharomyces cerevisiae 33800ppm. Hazardous characteristics: Flammable, its vapor and air can form explosive mixture. In case of open flame and high heat, it will cause combustion and explosion. Reacts violently in contact with oxidizing agents. In the event of a fire, there is a risk of explosion of heated containers. Its vapor is heavier than air, and it can spread to a considerable distance at a lower place, and it will cause flashback in case of an open flame.
Reference
1.Giuliani C., "The Flavonoid Quercetin Induces AP-1 Activation in FRTL-5 Thyroid Cells," Antioxidants, Vol.8, No.5(2019), p.112.
2.Xie L., Saimaier K. & Wang C. et al., "Methyl acetate arrests Th1 in peripheral immune system and alleviates CNS inflammation in EAE," International Immunopharmacology, Vol.101(2021), p.108291.
3.Maia A. C. D., Do Amaral Ferraz Navarro D. M. & Núñez-Avellaneda L. A. et al., "Methyl acetate, a highly volatile floral semiochemical mediating specialized plant-beetle interactions," The Science of Nature, Vol.108, No.3(2021).
4.Li Yufang and Wu Xiaoming: "Research Progress in my country's Acetate Synthesis of Methyl Acetate", "Fine and Specialty Chemicals", No. 04, 2022, pp. 44-46.
You may like
Related articles And Qustion
Lastest Price from Methyl acetate manufacturers

US $10.50/KG2025-06-06
- CAS:
- 79-20-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 ton
US $100.00/KG2025-04-21
- CAS:
- 79-20-9
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 200TON